Ventilator Associated Pneumonia in Adult Patients Preventive Measures: A Review of the Recent Advances
P Batra1, P Mathur1*, M. C. Misra2, M Kumari1, O Katoch1, F Hasan1
1JPNATC, Department of Lab Medicine, All India Institute of Medical Sciences, New Delhi, India
2Department of Surgery, Mahatma Gandhi University of Medical Sciences & Technology, Rajasthan, India
Abstract
Ventilator Associated Pneumonia (VAP) is the most commonly acquired ICU infection worldwide affecting nearly 10-30% of ventilated patients and accounting for nearly 25% of all types of ICU infections. VAP has been associated with increased morbidity, mortality, duration of ICU stay, duration of mechanical ventilation and nearly 50% of the ICU antibiotic prescription. After understanding the pathogenesis of VAP, various preventive measures have been tried by various authors. The currently accepted preventive measures are being used in most centres as the VAP prevention bundle. This includes: elevation of the head of the bed between 30oand 45o, daily sedation interruption and assessment of readiness to extubate, daily oral care with Chlorhexidine, peptic ulcer disease prophylaxis and deep vein thrombosis prophylaxis. In the current manuscript, we will be discussing the available preventive measures. Other measures which have been shown to be effective include selective oropharyngeal and digestive tract decontamination, use of antimicrobial coated ET tubes. However, more studies need to be done to see if these can be included in the VAP prevention bundle.
Introduction
Ventilator Associated Pneumonia (VAP) is the most commonly acquired ICU infection worldwide affecting nearly 10-30% of ventilated patients and accounting for nearly 25% of all types of ICU infections1. As defined by the US Centre for Disease Control and Prevention (CDC), it is pneumonia that develops 48 hours or more after the initiation of mechanical ventilation (MV)2. VAP has been associated with increased morbidity, mortality, duration of ICU stays, and nearly 50% of the ICU antibiotic prescription1. Understanding the pathogenesis of VAP is important to understand the various preventive measures available for prevention of VAP.
Pathophysiology of VAP3
Healthy individuals normally have various defence mechanisms to prevent the development of pneumonia. These include cough reflex, mucociliary clearance, anatomy of the airways and the presence of immunoglobulins, complement etc in the lower airways and alveoli. VAP occurs when bacteria are introduced into the normally sterile lower respiratory tract and overwhelm the host defence mechanisms. Disease causing bacteria enter the lower airways through endogenous or exogenous sources. The endogenous source includes aspiration of the bacteria colonising the upper airways or surrounding GI tract while exogenous source includes bacteria colonising or forming biofilm on the ET tube or the ventilator circuit. Detailed pathophysiology is given in (Figure 1).
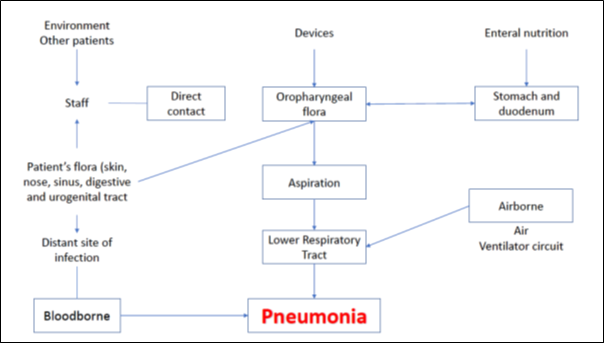
Figure 1: Routes of infection in ventilator associated pneumonia
VAP Preventive Measures
Any patient with MV is at risk of development of VAP. The risk of developing VAP is 3% per day during the first 5 days of MV, 2% per day for 6-10 days and 1% per day after that4. Thus, VAP prevention is best achieved by avoiding or minimising the duration of MV. Various strategies have been described to minimise this. These include:
Methods to reduce the time at risk for VAP development
VAP prevention begins with avoiding or limiting the duration of MV.
Non-invasive Positive Pressure Ventilation (NPPV): Many Randomised controlled trials (RCT) have concluded that the use of NPPV significantly reduces the risk of VAP development in comparison with the patients with invasive MV5. Studies have concluded that non-invasive ventilation is as effective as invasive ventilation in improving gas exchange and is also associated with fewer complications and adverse effects6. Therefore, it is recommended that NPPV be used whenever possible.
Daily weaning trials: Daily weaning trials have been repeatedly described and validated as strategies that limit the time of MV. Various RCTs have demonstrated that protocol directed sedation can reduce the duration of MV, ICU and hospital stay7.
Sedation vacation: Sedation vacation is an important component of VAP bundle. Based on the criterion of patient, sedation is decreased or interrupted in order to assess whether extubation is possible. If the criterion for extubation are met, patient is extubated. Study by Schweickert et al., demonstrated daily interruption of sedation reduced the duration of ICU stay, MV and incidence of complications8. This is called the Spontaneous Awakening Trial (SAT). In most of the studies conducted these days9, a protocol that pairs SAT with Spontaneous Breathing Trials (SBT) is confirmed to be most effective.
Reducing the chances of aspiration
Microorganisms reach the ETT either by aspiration of oropharyngeal colonised organisms or by gastroesophageal reflux secretions. Once the organisms reach ETT, they produce biofilm i.e., a community of bacteria which proliferate continuously and are protected from the host immune system and natural defences by the various chemicals produced10. These biofilms can dislodge subsequently and cause VAP. Various recent advances are available these days for VAP prevention in patients having ETT. These include:
Semirecumbent position: Clinical practise guidelines recommend elevation of the head end of bed by 30-45o to prevent aspiration of gastric contents11. Various studies have concluded that semirecumbency decreases the chances of pulmonary aspiration though it has no effect on the Gastro-Esophageal Reflux (GER). These studies have used instillation of radiolabelled compounds in gastric contents12,13. Rotating beds must be used in patients who are not able to tolerate semirecumbent position (such as patients in shock requiring high dose of ionotropes or patients with unstable spinal cord injury).
A multicentre prospective trial of ICU patients was conducted by Van Nieuwenhoven et al.,14 among patients receiving MV. The study compared patients in semirecumbent position (with a target backrest elevation of 45o) with the patients in supine position (with initial backrest elevation at 10o). The objectives of this study were to assess the feasibility of the semirecumbent position in mechanically ventilated patients and the effectiveness of the measure in VAP prevention. The target semirecumbent position of 45o was not achieved in 85% of the study time in the intervention group, being only 28.1 and 22.68 on average at days 1 and 7, respectively. Also, no significant difference was detected in the incidence of VAP which was 6.5% in the supine group and 10.7% in the semirecumbent group.
Thus, though there is a strong evidence that strict 0o supine position in patients with MV increases the risk of VAP, there is paucity of literature comparing 10-30o with 30-45o.
ETT with subglottic suctioning: Intermittent or continuous suctioning of the secretions that accumulate helps prevent microaspiration of the infected colonised secretions. Various studies15,16 have shown that continuous or intermittent drainage of accumulated secretions reduces the chances of VAP development, decreases the duration of MV and ICU stay. However, aspiration of the subglottic secretions has been associated with increased chances of damage to tracheal mucosa17. Changes in the design of ETT has reduced the chances of damage to tracheal mucosa but the cost has increased18. Studies have reported that the difference in the rate of VAP development by continuous or intermittent suctioning is not significant16. Thus, use of intermittent ET suctioning could decrease the risk of VAP development with only slight increase in the chances of tracheal mucosa damage.
Mucus shaver: Removal of ETT secretions is normally done by insertion of small flexible rubber catheter into the ETT. This method, however, is suboptimal as residual contaminated secretions are generally left behind which may lead to the formation of a biofilm. Mucus shavers, were thus designed to improve the cleaning process of the ETTs19. These are inflatable silicone rubber balloons to shave the lumen of the ETT.
Mucus slurper: This device was designed to remove the secretions of the proximal traches. It is a modifiable tracheal tube with 2 shaving rings that allows automatic aspiration of the secretions as it reaches the ETT20. The device is inserted into the distal portion of the ETT, inflated such that the shaver’s edge is in contact with the interior of the ETT, and then withdrawn over 3-6 sec to remove the accumulated secretions.
Reducing the endogenous source of infection
Selective digestive tract antimicrobial decontamination: Selective decontamination of digestive tract (SDD) use antimicrobial therapy to eradicate the potentially pathogenic microorganisms in the oral, gastric and intestinal tract. Antibiotics used are non-absorbable preparations of antibiotics with broad spectrum of activity administered enterally/parenterally/topical application21,22. The largest study was conducted in Netherlands in 13 ICUs showed a 28-day mortality reduction with the use of SDD and SOD by 3.5 and 2.9% respectively23. However, follow up study conducted by Oostdijk et al showed that SDD lead to increased bacterial resistance in the ICUs24.
Selective Oral Decontamination (SOD): Chlorhexidine is the most commonly used antiseptic agent for SOD for VAP prevention25. Its efficacy is dependent on the frequency of use and the percentage used (2% more effective than 0.12% or0.2%)26. Iseganan27 and povidone iodine28 are also being investigated for oral decontamination. Iseganan is a topical antimicrobial with activity against Gram-positive and gram-negative bacteria, and yeast. However, topical oropharyngeal administration failed to show any reduction in VAP when compared to placebo in a multicenter randomized trial. Povidone iodine has demonstrated a benefit in VAP rates in patients with severe head trauma, but this has yet to be investigated in other patient populations.
Use of probiotics29: Probiotics are living microorganisms that confer a health benefit when administered in adequate dosages. A 2010 pilot study found that critically ill patients at high risk for VAP who received Lactobacillus rhamnosus had significantly fewer microbiologically confirmed cases of VAP and significantly fewer episodes of Clostridium difficile-associated diarrhoea compared to patients who did not receive the probiotics. However, larger multi-center trials with more liberal inclusion criteria are needed to evaluate the generalizability of this finding.
Placement and modifications in the Endotracheal Tube
Appropriately inflated cuffed Endotracheal or tracheostomy tubes should be used in patients requiring MV. The cuff inflation pressure must be adjusted until there is no audible air leak. ETT cuff pressure of atleast 20 cm H2O must be maintained as per the American guidelines30. Currently there are two cuff shapes available for High Volume Low Pressure (HVLP) ETs with subglottic secretion. These can be spindle or tapered. The tapered cuff ETs were introduced as they were thought to have enhanced fit and also reduce the pressure impact on the trachea, thus causing lesser tracheal mucosa damage. However, the use of tapered tracheal cuff did not lead to a substantial reduction in the prevalence of VAP31.
Antimicrobial-coated endotracheal tubes: Use of silver coated ETTs, has been shown to reduce the biofilm production32-34. These studies have demonstrated the success of silver coated ETTs in reducing the rate of VAP, duration of MV and ICU stay. Though coated ETTs are expensive (90$ versus 2$ of uncoated tubes), but studies demonstrate significant reduction in the risk of VAP35. Silver sulphadiazine and chlorhexidine act directly on bacterial cell membrane causing distortion and enlargement of the cells, thus weaking the cell wall and cell membrane. Silver sulphadiazine dissociates upon exposure to bacterial surface. The dissociated silver moiety enters cell wall, attaches to the bacterial DNA and thus prevents cell proliferation. Chlorhexidine alters the cell membrane causing efflux of the nucleosides and nucleotides, and also facilitating the entry of silver sulphadiazine36.
In vitro animal studies of antiadhesive coating of ETT have also been performed since the biofilm formation starts with adhesion of the bacteria. These include, treatment of PVC with oxygen plasma, which produces a hydrophilic surface reducing the bacterial adhesion. Impregnation of the PVC with surfactants containing cholesterol and lecithin or other components of the innate system such as lactoferrin have also been investigated. However, antiadhesive treatment does not have antibacterial effect37.
Education
All health care providers involved in the care of patients on MV must be well educated in respect to the diagnosis and prevention of VAP as this has been shown to reduce the VAP rate38,39. However, studies have shown that despite all well-established guidelines, adherence to Evidence Based Guidelines (EBG) is poor among clinicians due to either unavailability of resources or cost or disagreement with the clinical trials40,41.
Hospital infection control policies11, 30
• Use standard precautions i.e., proper hand hygiene, use of appropriate PPE (eg gloves, goggles, face mask, gowns etc) etc for the care of mechanically ventilated patients to prevent person to person transmission of the infecting microorganisms.
• Transmission based precautions (contact, droplet, airborne) should be used when caring for patients colonised or infected with organisms spread by direct or indirect contact/droplet/airborne route.
• Cleaning of the critical care environment must be done regularly to prevent transmission of infective organisms from environment to patient
• Adherence to standard precautions esp hand hygiene must be monitored regularly
• Nebulisers and resuscitation equipment must be for single patient use. They should be changed in single patient when mechanically damaged or visibly soiled.
• Nosocomial surveillance for respiratory water pathogens must be done regularly
• Equipment sterilisation: All the reusable equipments to be used must be properly sterilised after thorough cleaning while the one time use equipments must be used only once strictly as per the manufacturer’s policy.
VAP Prevention Bundle42
A bundle is a small set of evidence based preventive practices that when implemented collectively helps in the prevention of a healthcare associated infection. The VAP prevention bundle adopted by most hospitals are those given by Institute of Healthcare Improvement. It includes: elevation of the head of the bed by 30o-45o, daily sedation interruption and assessment of readiness to extubate, daily oral care with Chlorhexidine, peptic ulcer disease prophylaxis (using sucrafate or H2 blockers43) and deep vein thrombosis prophylaxis. Though prophylaxis for peptic ulcer disease and deep vein thrombosis do not directly play role in the prevention of VAP.
Conclusion
Over the past few years, many new recent advances have been developed for the prevention of VAP based on the pathogenesis. Most of the above discussed recent advances have not been included in the VAP bundle. More studies are needed to assess the inclusion of these methods in the VAP prevention bundle.
References
- Batra P, Mathur P, John NV, et al. “Impact of multifaceted preventive measures on ventilator-associated pneumonia at a single surgical centre”. Intensive Care Medicine. 2015; 41(12): 2231-2232.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002; 165: 867– 903.
- Alcon A, Fabregas N, Torres A. Pathophysiology of Pneumonia. Clin Chest Med. 2005; 26: 39-46.
- Othman AA, Abdelazim MS. Ventilator-associated pneumonia in adult intensive care unit prevalence and complications. The Egyptian Journal of Critical Care Medicine. 2017; 5: 61-63.
- Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998; 339: 429–35.
- Hess DR. Noninvasive positive-pressure ventilation and ventilator-associated pneumonia. Respir Care. 2005; 50: 924–9. discussion 929-31.
- Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999; 27: 2609–15.
- Schweickert WD, Gehlbach BK, Pohlman AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004; 32: 1272–1276.
- Robertson TE, Mann HJ, Hyzy R, et al. Partnership for Excellence in Critical Care. Multicenter implementation of a consensus-developed, evidencebased, spontaneous breathing trial protocol. Crit Care Med. 2008; 36: 2753–2762.
- Berra L, De Marchi L, Yu ZX, et al. Endotracheal tubes coated with antiseptics decrease bacterial colonization of the ventilator circuits, lungs and endotracheal tube. Anesthesiology. 2004; 100: 1446–56.
- Tablan OC, Anderson LJ, Besser R, et al. Guidelines for Preventing Health-Care-Associated Pneumonia. 2003; 2004 / 53(RR03): 1-36.
- Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mehcnically ventilated patients: a randomized trial. Lancet. 1999; 354: 1851–8.
- van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006; 34: 396–402.
- van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associatedpneumonia: a randomized study. Crit Care Med. 2006; 34: 396–402.
- Ledgerwood LG, Salgado MD, Black H, et al. Tracheotomy tubes with suction above the cuff reduce the rate of ventilator-associated pneumonia in intensive care unit patients. Ann Otol Rhinol Laryngol. 2013; 122: 3–8.
- Muscedere J, Rewa O, McKechnie K, et al. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med. 2011; 39: 1985–91.
- Rello J, Soñora R, Jubert P, et al. Pneumonia in intubated patients: role of respiratory airway care. Am J Respir Crit Care Med. 1996; 154: 111–5.
- Youg PJ, Pakeerathan S, Blunt MC, et al. A low-volume, low-pressure tracheal tube cuff reduces pulmonary aspiration. Crit Care Med. 2006; 34: 632–9.
- Kolobow T, Berra L, Li Bassi G, et al. Novel system for complete removal of secretions within the endotracheal tube: the Mucus Shaver. Anesthesiology. 2005; 102: 1063-5.
- Kolobow T, Li Bassi G, Curto F, et al. The Mucus slurper: a novel tracheal tube that requires no tracheal tube suctioning. A preliminary report. Intensive Care Med. 2006; 32: 1414-8.
- de Smet AM, Bonten MJ. Selective decontamination of the digestive tract. Curr Opin Infect Dis. 2008; 21: 179–83.
- Silvestri L, van Saene HK, Milanese M, et al. Selective decontamination of the digestive tract reduces bacterial bloodstream infection and mortality in critically ill patients. Systematic review of randomized, controlled trials. J Hosp Infect. 2007; 65: 187–203.
- De Smet AM, Kluytmans JA, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009; 360: 20–31.
- Oostdijk EA, de Smet AM, Blok HE, et al. Ecologicalc effects of selective decontamination on resistant gram-negative bacterial colonization. Am J Respir Crit Care Med. 2010; 181: 452–7.
- Özçaka Ö, Ba?o?lu OK, Buduneli N, et al. Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: a randomized clinical trial. J Periodontal Res. 2012; 47: 584–92.
- Tantipong H, Morkchareonpong C, Jaiyindee S, et al. Randomized controlled trial and meta-analysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator associated pneumonia. Infec Control Hosp Epidemiol. 2008; 29: 131–6.
- Kollef MH, Pittet D, Sanchez Garcia M, et al. A randomized double-blind trial of iseganan in prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006; 173: 91–7.
- Seguin P, Tanguy M, Laviolle B, et al. Effect of oropharyngeal decontamination by povidone-iodine on ventilator-associated pneumonia in patients with head trauma. Crit Care Med. 2006; 34: 1514–9.
- Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010; 182: 1058–64.
- Oliveira J, Zagaloa C, Cavaco-Silvaa P. Prevention of ventilator-associated pneumonia. Rev Port Pneumol. 2014; 20(3): 152---161.
- Karchmer TB, Giannetta ET, Muto CA, et al. A randomized crossover study of silver-coated urinary catheters in hospitalized patients. Arch Intern Med. 2000; 160: 3294–8.
- Mahmoodpoor A, Hamishehkar H, Hamidi M, et al. A prospective randomized trial of tapered-cuff endotracheal tubes with intermittent subglottic suctioning in preventing ventilator-associated pneumonia in critically ill patients. Journal of Critical Care. 2017; 38: 152–156.
- Ahearn DG, Grace DT, Jennings MJ, et al. Effects of hydrogel/silver coatings on in vitro adhesion to catheters of bacteria associated with urinary tract infections. Curr Microbiol. 2000; 41: 120–5.
- Kollef MH, Afessa B, Anzueto A, et al. Silver-coated endotracheal tubes and the incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008; 300: 805–13.
- Shorr AF, Zilberberg MD, Kollef M. Cost-effectiveness analysis of a silver-coated endotracheal tube to reduce the incidence of ventilator-associated pneumonia. Infect Control Hosp Epidemiol. 2009; 30: 759–63.
- Chaiban G, Hanna H, Dvorak T, et al. A rapid method of impregnating endotracheal tubes and urinary catheters with gendine: a novel antiseptic agent. J Antimicrob Chemother. 2005; 55: 51–6.
- Berra L, Sampson J, Fumagalli J, et al. Alternative approaches to ventilator associated pneumonia prevention. Minerva Anestesiologica. 77(3): 323-333.
- Bloos F, Muller S, Harz A, et al. Effects of staff training on the care of mechanically ventilated patients: a prospective cohort study. Br J Anaesth. 2009; 103: 232–7.
- Salahuddin N, Zafar A, Sukhyani L, et al. Reducing ventilator associated pneumonia rates through a staff education programme. J Hosp Infect. 2004; 57: 223–7.
- Rello J, Lorente C, Bodi M, et al. Why do physicians not follow evidence based guidelines for preventing ventilator-associated pneumonia? A survey based on the opinions of an international panel of intensivists. Chest. 2002; 122: 656–61.
- Ricart M, Lorente C, Diaz E, et al. Nursing adherence with evidence-based guidelines for preventing ventilator-associated pneumonia. Crit Care Med. 2003; 31: 2693–96.
- Institute for Healthcare Improvement. Implement of IHIVentilator Bundle; 2013. Available from: http://www.ihi.org/knowledge/Pages/Changes/ImplementtheVentilato\rBundle.aspx
- Kallet RH, Quinn TE. The Gastrointestinal Tract and Ventilator-Associated Pneumonia. Respiratory Care. 2005; 50 (7): 910-921.
